Beckmann Rearrangement
The acid-catalyzed conversion of ketoximes to N-substituted amides is known as Beckmann Rearrangement.
The reaction is catalysed by acidic reagents such as, H2SO4, SOCl2, SO3,P2O5, PCl5, C6H5SO2 Cl, etc.
The reaction involves the migration of a group from carbon to electron-deficient nitrogen.

Some aldoximes undergo the rearrangement in the presence of polyphosphoric acid (PPA) but the reaction is not a general one. The migration of the group depends not on the migrational aptitude but upon the orientation of the group in relation to the OH group. It is found that the migrating group is always anti (i.e., trans) to the hydroxyl group. Thus, the reaction is stereospecific.

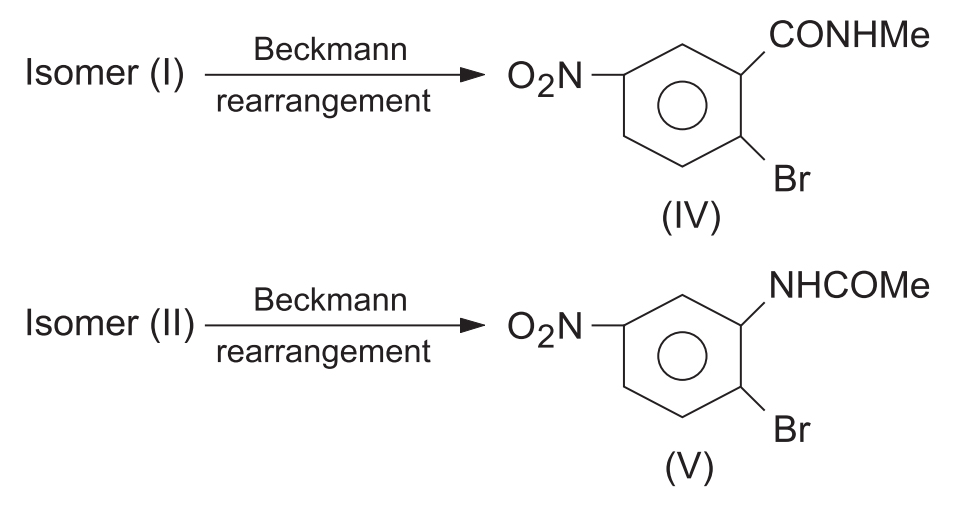
That it is always the antigroup which migrates has been confirmed by the rearrangements of the two isomeric oximes of 2-bromo-5-nitroacetophenone. The structures of the two isomeric oximes were first determined by an elegant method as given below.
On treatment with cold NaOH solution, one isomer (I) was cyclized to 3-methyl-5-nitrophenyl isooxazole (III) while the other isomer (II) remained unaffected even under drastic conditions.

Obviously, the OH and Br groups in isomer (I) are close enough for reaction and cyclization. Hence, the Me and OH groups are anti (i.e., trans) to each other. In isomer (II), the OH and Br groups are far apart for reaction, i.e., the Me and OH groups are syn (i.e., cis) to each other. Thus, the structures of isomers (I) and (II) are confirmed.
Now, on subjecting the two isomers to Beckmann rearrangement it is found that (a) isomer (I) gives N-methyl-2-bromo-5-nitrobenzamide (IV) indicating the migration of the antigroup Me to the nitrogen atom and (b) isomer (II) gives N-(2-bromo-5-nitrophenyl)- acetamide (V) due to the migration of the anti-aryl group to the nitrogen atom.
Oxime esters and ethers also undergo Beckmann rearrangement. The acidic reagents convert the OH group to a better leaving group—acids convert OH to H2O, other reagents convert OH to an ester-leaving group, e.g., OPCl4 from PCl5, OSO2C6H5 from C6H5SO2Cl, etc. The reaction is facilitated by heat, polar solvents or an increase in the acid strength.
That direct interchange of the migrating group and OH does not occur is proved by the fact that 18O is incorporated in the product in the presence of H218O.

Mechanism of Beckmann Rearrangement
The mechanism of the reaction is as given below.

With other acidic reagents, e.g., PhSO2Cl, the same intermediate (VI) is obtained.

In strong acids, the reaction proceeds with the protonation of the OH group of the oxime with subsequent loss of water to yield the species (VI) with electron-deficient nitrogen which is also obtained with other acidic reagents by the loss of ester group. The migration of R then gives a carbocation. The attack of water molecule on the carbon followed by the loss of proton gives the amide.
The migrating group retains its configuration and hence the migrating group does not become completely free during the migration, otherwise the reaction cannot be stereospecific. Thus, the migration and the breaking of N–O bond may be concerted or at least very rapid.
Applications of Beckmann Rearrangement
1. Configuration of ketoximes can be assigned
A ketoxime gives an amide on Beckmann rearrangement. From the products of hydrolysis of the amide, the structure of the amide is known and for that matter, the configuration of the oxime is known.
Thus,

Formation of RNH2 indicates the migration of the group R to the nitrogen atom. The groups R and OH are, therefore, anti to each other, i.e., the structure of the oxime is

2. Synthesis of isoquinoline

3. Synthesis of lactams
Alicyclic ketones of all ring sizes undergo the Beckmann rearrangement of their oximes to yield lactams.
4. Synthesis of paracetamol
It is used in the industries for the synthesis of paracetamol. This integration is achieved by the process of conversion of a ketone to ketoxime with the help of hydroxylamine.
